
量子计算 +AI,打破药物研发瓶颈?

英矽智能结合量子计算与 AI,突破 KRAS 靶点药物研发瓶颈
KRAS 是癌症中极为常见的 “问题蛋白”,在大约四分之一的人类肿瘤中都存在 KRAS 突变。这种突变会让细胞无限制增殖,从而引发癌症。虽然相关突变非常普遍且危害巨大,但目前被 FDA 批准的、专门针对 KRAS 突变的药物仅有两种,而且它们只能在一定程度上延长患者的生存期。对很多癌症患者来说,亟需能带来更大获益的新型 KRAS 疗法。
在近期发表于Nature Biotechnology的研究中,来自英矽智能(Insilico Medicine)与加拿大多伦多大学、以及其他科研机构如圣裘德儿童研究医院等,展开了一次 “量子计算 + 经典计算 + 生成式 AI” 的跨界合作,尝试从头设计出对付 “不可成药” KRAS 的新型抑制剂分子。
该研究首次展示了量子计算结合 AI 在药物早期发现过程中的潜在优势,为高难度靶点的治疗方案带来了新的希望。
量子计算 +AI,如何构建药物分子生成过程
研究团队提出了一种量子 - 经典混合的生成框架:结合 ** 量子变分生成模型(QCBM)和长短期记忆网络(LSTM)** 来协同设计新分子。具体而言,他们用一个包含 110 万种分子的定制数据集对量子 - 经典混合模型进行 “训练”。这个庞大的数据来源包括:
- 650 个已在文献中被证实可阻断 KRAS 的分子,
- 使用 STONED-SELFIES 算法在已知 KRAS 抑制剂基础上衍生出的 85 万种类似物,
- 通过虚拟筛选平台 VirtualFlow 获取的 25 万种分子。
如此丰富的训练数据,让量子 - 经典混合模型学到更广阔的 “化学空间”,为后续生成多样化的候选分子奠定了基础。
接下来,就是量子与经典的协同生成过程。
在这套量子 - 经典混合模型中,
- QCBM:充当量子生成模型,利用量子电路来学习复杂的概率分布,生成与训练数据相似却 “尚未被探索” 的分子结构。它同时还充当 “先验(prior)”,引导 LSTM 的分子序列生成。
- LSTM:则发挥经典 AI 模型的优势,能理解并生成序列数据。通过引入 QCBM 输出的概率分布,LSTM 在生成新的化学结构时可以更精准地把握分子多样性,避免过度拟合或收敛于熟悉的结构。
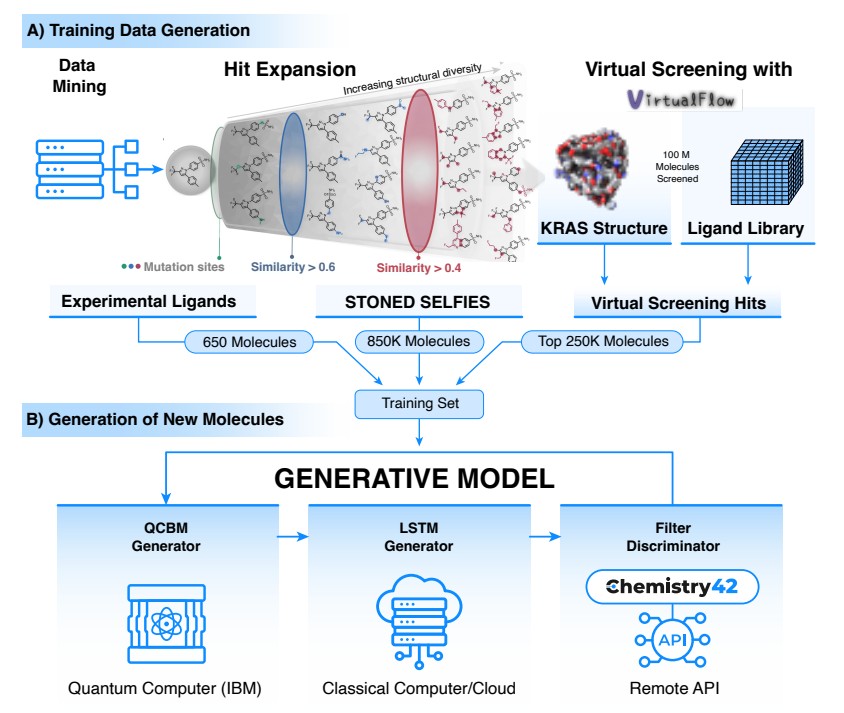
在实际应用中,研究团队先用混合模型一次性产生了 100 万种候选分子。接着,他们借助英矽智能自主研发的Chemistry42生成式人工智能引擎,对这些分子进行系统的评估和筛选,包括类药性、对接评分、合成可及性等多个维度,从中挑选出 15 种最具潜力的候选分子进入实验室测试。
从云端筛选到实验验证
与传统药物发现相比,这种方法不需要依赖大规模的物理化合物库来进行昂贵、冗长的高通量筛选。相反,大部分筛选工作都能在云端完成,大大降低成本与时间。而在最终实验室阶段,针对那 15 种优选分子进行了 “湿实验” 测试,结果发现 2 个分子格外突出。
其中,名为ISM061-018-2的分子既有较强的靶向 KRAS 活性,又未表现出明显的细胞毒性。同时,它对野生型 KRAS 和多种常见突变型 KRAS(以及野生型 HRAS、NRAS)都具有抑制活性,展现出成为 “泛 RAS 抑制剂” 的潜力。
另外一个分子ISM061-022则在针对某些突变型 KRAS(如 G12R、Q61H)上表现出更高效的抑制作用,也同样有望发展成为广谱抗癌药物的候选。
值得注意的是,目前的研究还无法证明这类量子 - 经典混合方法比纯经典方法 “更优”,但至少说明量子计算在药物早期发现中具备可行性和潜在加速作用。随着量子计算硬件的不断升级,其在生成式模型中的应用前景也会相应扩大。
多伦多大学化学与计算机科学教授Alán Aspuru-Guzik博士表示:“这是一次原理验证的研究,它初步表明量子计算机能够融入现代 AI 驱动的药物研发流程,并成功设计出能与生物靶标结合的活性分子。尽管目前还没看到 ‘量子计算对经典方法的绝对优势’,但随着量子硬件能力增强,我们期望相关算法会越来越 ‘显威’。”
在获得了针对 KRAS 成功的早期成果后,研究团队计划把这套量子 - 经典混合模型推广到更多 “不可成药” 的蛋白靶点上。与 KRAS 类似,这些蛋白小巧、表面缺乏能与化合物稳定结合的 “口袋”,一直是药物研发中最棘手的目标之一。研究人员还将继续优化已经获得的 KRAS 苗头化合物,并在动物模型中进行验证,力求为癌症患者带来更有效的新一代分子。
英矽智能创始人兼 CEO Alex Zhavoronkov博士表示:“多达 85% 的人类蛋白质被认为是 ‘不可成药’ 的,如何从这些蛋白中 ‘开拓出可能性’,一直是抗癌研究的挑战所在。人工智能刚好能在这块难啃的骨头上展现独特的力量。我们非常高兴能与多伦多大学携手,把量子计算融入 AI 驱动的药物发现流程,为人类健康谋求更多的可能。”
量子与 AI 融合前景广阔
事实上,这并非英矽智能与多伦多大学的首次 “联手”。早在 2023 年,他们就在Journal of Chemical Information and Modeling上发表了第一篇合作论文,通过多个实验场景将变分量子线路(VQC)逐步取代经典生成模型 MolGAN 的不同部分,探讨量子生成对抗网络在小分子药物发现中的应用。
本次最新发表于 Nature Biotechnology的成果,再次印证了量子计算在药物设计阶段拥有的潜在价值。随着量子计算技术与 AI 生成式模型的进一步结合,未来或许能更快、更精准地筛选到针对那些 “疑难靶点” 的活性分子,为更多患者带去希望。
虽然目前还不能断言量子计算已经超越经典算法,但英矽智能作为 AI 制药领域的先行探索者,正积极寻求量子计算与 AI 相结合的方式,以便在量子计算技术获得突破时取得先发优势。 这一思路也与国际上对于 AI 药物研发的乐观预期相呼应。
诺奖得主兼 Google DeepMind 首席执行官 Demis Hassabis 近日在达沃斯世界经济论坛上表示,今年底前,基于 AI 设计的药物有望进入临床试验。
这些药物由 Alphabet 旗下 Isomorphic Labs 研发,旨在从第一性原理重塑药物发现流程。Hassabis 指出,AlphaFold 技术已成功预测 2 亿种蛋白结构,为精准研发提供了前所未有的可能。
在这一背景下,更多科研机构、初创企业和大型跨国药企都在持续探索 AI 与量子计算等前沿技术的结合。AI不仅能够帮助科学家从庞大的分子空间中快速筛选潜在候选物,还可进一步借助量子计算的强大算力,寻找更符合 “不可成药” 靶点需求的新分子设计思路。

